Biologia plantarum 69:106-117, 2025 | DOI: 10.32615/bp.2025.011
Comparative alterations in root cell wall constituents and cation-exchange capacity of two tomato cultivars under salinity induced by NaCl and CaCl2
- 1 Department of Soil Science, Faculty of Agriculture, University of Calabar, P.M.B. 1115, Calabar, Nigeria
- 2 Arid Land Research Centre, Tottori University, Hamasaka, Tottori 680-0001, Japan
- 3 Department of Crop Science, Faculty of Agriculture, University of Calabar, P.M.B. 1115, Calabar, Nigeria
- 4 Department of Plant and Environmental Sciences, Clemson University, Clemson, SC 29634, USA
- 5 Center for Agricultural Resources Research, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, 268 Huaizhong Road, Shijiazhuang, Hebei Province, 050023, P.R. China
Background: Tomato plants exposed to salinity stress may experience dynamic changes in root growth and cell wall (CW) composition and structure.
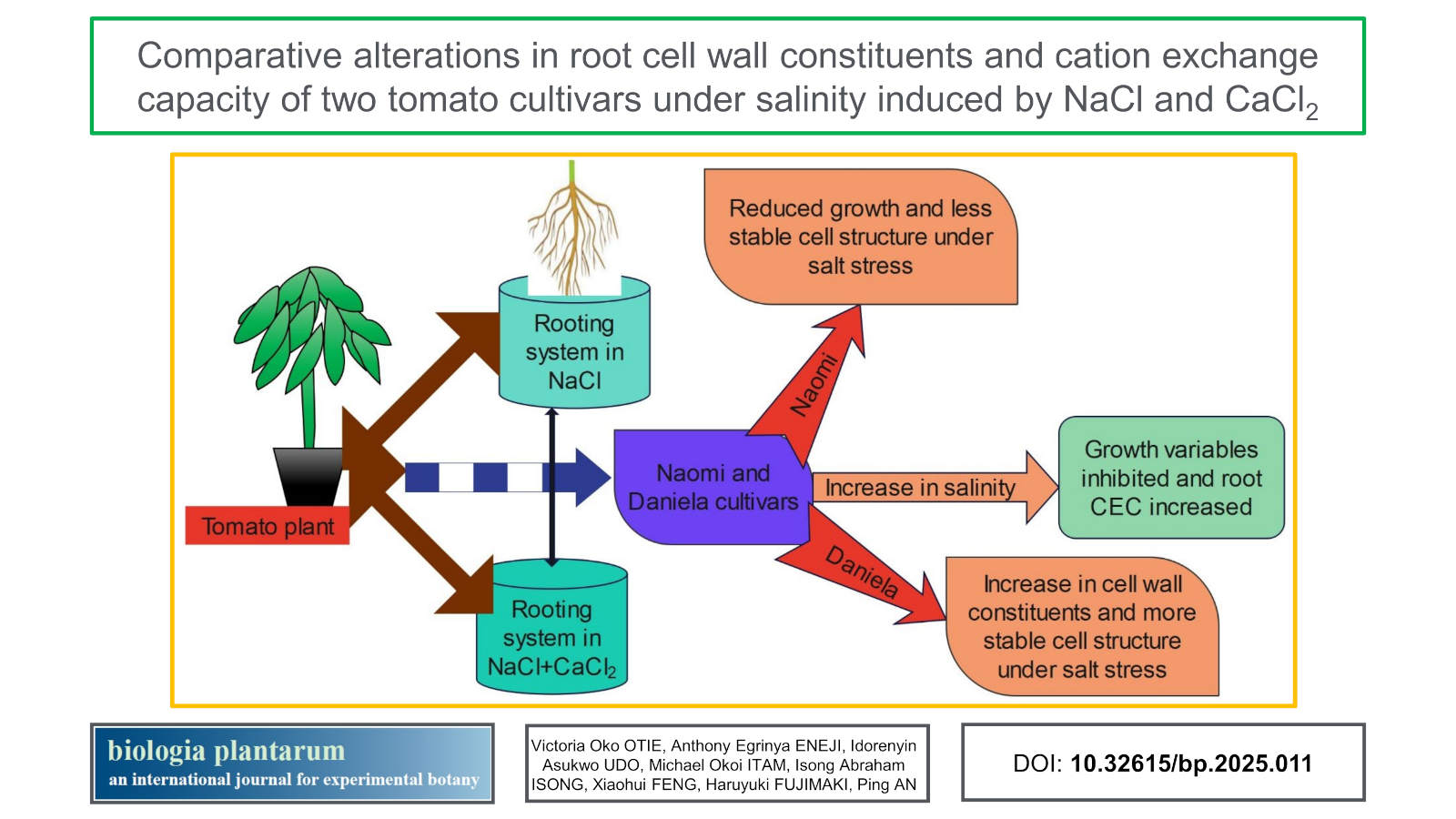
Aims: Here, we determined the CW composition and cation-exchange capacity (CEC) of two tomato cultivars (Daniela, salt-tolerant and Naomi, salt-sensitive) as well as their growth and root characteristics.
Methods: Seedlings of the tomato cultivars were exposed to six NaCl plus CaCl2 concentrations hydroponically, root growth and CW chemical composition were measured.
Results: The root growth of Naomi was adversely (P ≤ 0.05) reduced at the elongation zone, but there was little change in the chemical composition of the CW under salinity. A marked reduction occurred in the CW-constituting polysaccharides of Naomi relative to Daniela, whether at the 8.00 dS m-1 NaCl treatment or its combination with CaCl2. For both root zones, CW viscosity was better enhanced under NaCl and CaCl2 combinations, but the contents of uronic acid across the CW constituents increased under sole treatment with CaCl2 at the mature root zone of Naomi. The root CW CEC increased (P ≤ 0.05) with increases in the ionic concentration of the external solution. Salt concentrations at 8.0 dS m-1 NaCl or 8.0 dS m-1 NaCl + CaCl2 increased (P ≤ 0.05) the CEC of the CW, especially for Daniela.
Conclusions: The overall results showed that CaCl2 could enhance some tolerance in CW polysaccharides of tomato under salinity stress. The salt-tolerant Daniela with higher CW and ionic contents had superior stability in cell structure under salt stress.
Keywords: Lycopersicon esculentum L., salinity stress, salt tolerance, uronic acid.
Received: May 31, 2025; Revised: September 11, 2025; Accepted: November 24, 2025; Published online: February 5, 2026 Show citation
| ACS | AIP | APA | ASA | Harvard | Chicago | Chicago Notes | IEEE | ISO690 | MLA | NLM | Turabian | Vancouver |
References
- Alam, M.S., Tester, M., Fiene, G. & Mousa, M.A.A. (2021) Early growth stage characterization and the biochemical responses for salinity stress in tomato. Plants, 10, 712.
 Go to original source...
Go to original source... - Albersheim, P., Darvill, A., Roberts, K., Sederoff, R. & Staehelin, A. (Eds.) (2010) Plant Cell Walls: From Chemistry to Biology. New York: Garland Science, pp. 430.
 Go to original source...
Go to original source... - An, P., Inanaga, S., Li, X.J., Eneji, A.E. & Zhu, N.W. (2005) Interactive effects of salinity and air humidity on two tomato cultivars differing in salt tolerance. Journal of Plant Nutrition, 28, 459-473.
 Go to original source...
Go to original source... - An, P., Li, X., Zheng, Y. et al. (2014) Effects of NaCl on root growth and cell wall composition of two soya bean cultivars with contrasting salt tolerance. Journal of Agronomy and Crop Science, 200, 212-218.
 Go to original source...
Go to original source... - Annunziata, M.G., Ciarmiello, L.F., Woodrow, P., Maximova, E., Fuggi, A. & Carillo, P. (2017) Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Frontiers in Plant Science, 7, 2035.
 Go to original source...
Go to original source... - Blumenkrantz, N. & Asboe-Hansen, G. (1973) New method for quantitative determination of uronic acids. Analytical Biochemistry, 54, 484-489.
 Go to original source...
Go to original source... - Byrt, C.S., Munns, R., Burton, R.A., Gilliham, M. & Wege, S. (2018) Root cell wall solutions for crop plants in saline soils. Plant Science, 269, 47-55.
 Go to original source...
Go to original source... - Cheeseman, J.M. (2015) The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytologist, 206, 557-570.
 Go to original source...
Go to original source... - Choi, W.-G., Toyota, M., Kim, S.-H., Hilleary, R. & Gilroy, S. (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proceedings of the National Academy of Sciences of the United States of America, 111, 6497-6502.
 Go to original source...
Go to original source... - Corrêa-Ferreira, M.L., Viudes, E.B., de Magalhães, P.M. et al. (2019) Changes in the composition and structure of cell wall polysaccharides from Artemisia annua in response to salt stress. Carbohydrate Research, 483, 107753.
 Go to original source...
Go to original source... - Cosgrove, D.J. (2016) Catalysts of plant cell wall loosening. F1000Research, 5, 119.
 Go to original source...
Go to original source... - Endler, A. & Persson, S. (2011) Cellulose synthases and synthesis in Arabidopsis. Molecular Plant, 4, 199-211.
 Go to original source...
Go to original source... - Feng, W., Kita, D., Peaucelle, A. et al. (2018) The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Current Biology, 28, 666-675.
 Go to original source...
Go to original source... - Guo, M., Wang, X.-S., Guo, H.-D. et al. (2022) Tomato salt tolerance mechanisms and their potential applications for fighting salinity: a review. Frontiers in Plant Science, 13, 949541.
 Go to original source...
Go to original source... - Guo, Y., Liu, Y., Zhang, Y. et al. (2021) Effects of exogenous calcium on adaptive growth, photosynthesis, ion homeostasis and phenolics of Gleditsia sinensis Lam. plants under salt stress. Agriculture, 11, 978.
 Go to original source...
Go to original source... - Hocq, L., Pelloux, J. & Lefebvre, V. (2017) Connecting homogalacturonan-type pectin remodeling to acid growth. Trends in Plant Science, 22, 20-29.
 Go to original source...
Go to original source... - Hodge, J.E. & Hofreiter, B.T. (1962) Determination of reducing sugars and carbohydrates. In: Whistler, R.L. & Wolfrom, M.L. (Eds.): Methods in Carbohydrate Chemistry. New York: Academic Press, pp. 380-394.
- Huqe, M.A.S., Haque, M.S., Sagar, A. et al. (2021) Characterization of maize hybrids (Zea mays L.) for detecting salt tolerance based on morpho-physiological characteristics, ion accumulation and genetic variability at early vegetative stage. Plants, 10, 2549.
 Go to original source...
Go to original source... - Ismail, A.M. & Horie, T. (2017) Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annual Review of Plant Biology, 68, 405-434.
 Go to original source...
Go to original source... - Lamers, J., van der Meer, T. & Testerink, C. (2020) How plants sense and respond to stressful environments. Plant Physiology, 182, 1624-1635.
 Go to original source...
Go to original source... - Le Gall, H., Philippe, F., Domon, J.-M., Gillet, F., Pelloux, J. & Rayon, C. (2015) Cell wall metabolism in response to abiotic stress. Plants, 4, 112-166.
 Go to original source...
Go to original source... - Liu, H., Pan, L., Ahmad, I. et al. (2025) Study on the mechanism of exogenous CaCl2 regulating cell growth and development to alleviate salt tolerance of alfalfa (Medicago sativa). Frontiers in Plant Science, 16, 1565723.
 Go to original source...
Go to original source... - Liu, J., Otie, V., Matsuura, A. et al. (2022a) Pectin characteristics affect root growth in spinach under salinity. Plants, 11, 3130.
 Go to original source...
Go to original source... - Liu, J., Shao, Y., Feng, X. et al. (2022b) Cell wall components and extensibility regulate root growth in Suaeda salsa and Spinacia oleracea under salinity. Plants, 11, 900.
 Go to original source...
Go to original source... - Loix, C., Huybrechts, M., Vangronsveld, J., Gielen, M., Keunen, E. & Cuypers, A. (2017) Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Frontiers in Plant Science, 8, 1867.
 Go to original source...
Go to original source... - Meychik, N., Nikolaeva, Y. & Kushunina, M. (2021) The significance of ion-exchange properties of plant root cell walls for nutrient and water uptake by plants. Plant Physiology and Biochemistry, 166, 140-147.
 Go to original source...
Go to original source... - Meychik, N.R., Lyubimova, E.G. & Yermakov, I.P. (2010a) Ion-exchange properties of the cell wall of reindeer lichen Cladonia rangiferina. Russian Journal of Plant Physiology, 57, 260-266.
 Go to original source...
Go to original source... - Meychik, N.R., Nikolaeva, J.I. & Yermakov, I.P. (2005) Ion exchange properties of the root cell walls isolated from the halophyte plants (Suaeda altissima L.) grown under conditions of different salinity. Plant and Soil, 277, 163-174.
 Go to original source...
Go to original source... - Meychik, N.R. & Yermakov, I.P. (2001) Ion exchange properties of plant root cell walls. Plant and Soil, 234, 181-193.
 Go to original source...
Go to original source... - Meychik, N.R., Yermakov, I.P., Khonarmand, S.D. & Nikolaeva, Y.I. (2010b) Ion-exchange properties of cell walls in chickpea cultivars with different sensitivities to salinity. Russian Journal of Plant Physiology, 57, 620-630.
 Go to original source...
Go to original source... - Meychik, N.R., Yermakov, I.P. & Savvateeva, M.V. (1999) Ionogenic groups in the cell wall of wheat roots. Russian Journal of Plant Physiology, 46, 742-747.
- Munns, R., Day, D.A., Fricke, W. et al. (2020) Energy costs of salt tolerance in crop plants. New Phytologist, 225, 1072-1090.
 Go to original source...
Go to original source... - Munns, R. & Tester, M. (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651-681.
 Go to original source...
Go to original source... - Otie, V., Ibrahim, A., Udo, I. et al. (2022) Foliarly applied 24-epibrassinolide modulates the electrical conductivity of the saturated rhizospheric soil extracts of soybean under salinity stress. Plants, 11, 2330.
 Go to original source...
Go to original source... - Peaucelle, A., Braybrook, S. & Höfte, H. (2012) Cell wall mechanics and growth control in plants: the role of pectins revisited. Frontiers in Plant Science, 3, 121.
 Go to original source...
Go to original source... - Rahman, M.M., Mostofa, M.G., Keya, S.S. et al. (2021) Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. International Journal of Molecular Sciences, 22, 10733.
 Go to original source...
Go to original source... - Rana, V., Ram, S., Sendhil, R., Nehra K. & Sharma, I. (2015) Physiological, biochemical and morphological study in wheat (Triticum aestivum L.) RILs population for salinity tolerance. Journal of Agricultural Science, 7, 119-128.
 Go to original source...
Go to original source... - Raza, M.A., Saeed, A., Munir, H. et al. (2017) Screening of tomato genotypes for salinity tolerance based on early growth attributes and leaf inorganic osmolytes. Archives of Agronomy and Soil Science, 63, 501-512.
 Go to original source...
Go to original source... - Reina-Sánchez, A., Romero-Aranda, R. & Cuartero, J. (2005) Plant water uptake and water efficiency of greenhouse tomato cultivars irrigated with saline water. Agricultural Water Management, 78, 54-66.
 Go to original source...
Go to original source... - Rozema, J. & Schat, H. (2013) Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environmental and Experimental Botany, 92, 83-95.
 Go to original source...
Go to original source... - Shabala, S. (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany, 112, 1209-1221.
 Go to original source...
Go to original source... - Sivakumar, J., Prashanth, J.E.P., Rajesh, N., Reddy, S.M. & Pinjari, O.B. (2020) Principal component analysis approach for comprehensive screening of salt stress-tolerant tomato germplasm at the seedling stage. Journal of Biosciences, 45, 141.
 Go to original source...
Go to original source... - Tanveer, K., Gilani, S., Hussain, Z., Ishaq, R., Adeel, M. & Ilyas, N. (2019) Effect of salt stress on tomato plant and the role of calcium. Journal of Plant Nutrition, 43, 28-35.
 Go to original source...
Go to original source... - Uddin, M.N., Hanstein, S., Leubner, R. & Schubert, S. (2013) Leaf cell-wall components as influenced in the first phase of salt stress in three maize (Zea mays L.) hybrids differing in salt resistance. Journal of Agronomy and Crop Science, 199, 405-415.
 Go to original source...
Go to original source... - Volkov, V. (2015) Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to generic and protein engineering for manipulating ion fluxes. Frontiers in Plant Science, 6, 873.
 Go to original source...
Go to original source... - Zaki, H.E.M. & Yokoi, S. (2016) A comparative in vitro study of salt tolerance in cultivated tomato and related wild species. Plant Biotechnology, 33, 361-372.
 Go to original source...
Go to original source... - Zhang, S.-S., Sun, L., Dong, X., Lu, S.-J., Tian, W. & Liu, J.-X. (2016) Cellulose synthesis genes CESA6 and CSI1 are important for salt stress tolerance in Arabidopsis. Journal of Integrative Plant Biology, 58, 623-626.
 Go to original source...
Go to original source... - Zhao, C., Zayed, O., Zeng, F. et al. (2019) Arabinose biosynthesis is critical for salt stress tolerance in Arabidopsis. New Phytologist, 224, 274-290.
 Go to original source...
Go to original source... - Zhong, H. & Lauchli, A. (1993) Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. Journal of Experimental Botany, 44, 773-778.
 Go to original source...
Go to original source...



